Diagnostic Medical Physics
- Radiography, Fluoroscopy and Mammography
- Computed Tomography
- Ultrasound
- Magnetic Resonance
- Modality comparison, image features and artifacts
- Endogenous and exogenous contrast
- Modality facility considerations, safety
- Methods of quality control and quality assurance
Radiography, Fluoroscopy and Mammography
Modalities using planar x-rays. Contrast comes from the attenuation of x-rays by the body as they pass from the x-ray tube though material to a detector.
Expand x-ray modality details
X-ray principles
X-ray tube has most component in a vacuum (don't want electrons interacting with air)
Electrons generated in cathode with low voltage boiling electrons from filament. High voltage electric potential accelerate electrons to anode. Negatively charged focusing cup used to focus electrons. Filament size determines spot size (usually have two sizes). Anode spins to spread out heat. Cathode to anode in vacuum and cooled with oil.
Energy deposited in anode: $E(J) = mAs \times kVp$
Anode angle alters true focal spot to an effective focal spot: $l_{eff} = l_t\sin(\theta)$
Angle for imaging is usually 6-17 degrees, and for therapy is ~30 degrees
Focal spot blur: occurs because spot is not a point.
$\text{blur} = F \times OID / SOD $ where F is size of spot, OID is object image distance, SOD is source object distance
Heel effect - target self-absorption. Intensity reduced from cathode to anode.
AC voltage need rectification to produce current consistently (otherwise anode positive only half the time).
Voltage ripple: $(V_{min} - V_{max}) / V_{max} * 100$
Three phase generators: size pulses per cycle, 13-25% ripple
Constant potential generators: uses rectifiers, capacitors, and triode valve, <2% ripple
High-frequency generator rectified and smoothed (via capacitors) and fed to a chopper and inverter circuits, <2% ripple
Fluorescent/characteristic and bremsstrahlung x-rays produced. See atomic/nuclear physics page for details
Fluorescent/characteristic x-rays: photoelectric effect, electron capture/internal conversion, heavy ions
Energy of fluorescence depend on binding energy: $E = h\nu - BE$
Energy proportional to Z, and Z linear with sqrt of frequency for K and L series.
Fluorescence yield is probability an x-ray will escape an atom. About 0 for Z < 10, ~ 0.95 for Z = 74 (W)
Isotropically emitted
Characteristic x-rays exploited mostly in mammography
Bremsstrahlung: photons emitted from accelerating charged particles (deflected by nucleus of atom)
Any amount may be emitted up to the energy of the charged particle → continuous energy spectrum
Probability is proportional to Z2 and to 1/me (i.e. very limited for particles heavier than electrons).
Efficiency is energy emitted by photons / energy of deposited by electrons: $\epsilon = 9 \times 10^{-10} ZV$ where $Z$ is atomic number and $V$ is tube voltage. For typical imaging devices, $\epsilon < 1%$
As electron energy increases, photons are emitted in more forward direction
Quality: characterizes beam spectrum, i.e. energy, penetrating power, hardness. Often expressed in HVL
Typically use aluminum (0.5 - 1 mm) to filter out low energy bremsstrahlung x-rays (that won't pass through object to be included in image).
Hardens beam, but need to balance hardness and intensity (more filtering lowers intensity).
Use higher Z material (e.g., copper) for pediatric or thin patients.
X-ray output exposure is proportional to current, time, and peak voltage squared: mAs and kVp2.
Radiography
Magnification: M = source to image / source to object
Diagnostic x-ray exposure limit at 1 m: 100 mR/hr
Optical density: darkness of film after radiation exposure. Proportional to the log of the ratio of intensity of transmitted light relative to intensity of unexposed film.
$\text{OD} = -\log(I/I_0) = \log(I_0/I)$
Fluoroscopy
Barium swallow - to check GI tract (dysphagia, hiatal hernia, GERD, ulcers, tumors, etc.)
Mammography
Uses Rh and Mo targets and filters to take advantage of characteristic x-rays in 20-40 keV range (good image contrast and penetrating enough for breast).
Mo - 17.5, 19.6 keV
Rh - 20.2 keV, 22.7 keV
Mo target, Mo filter - good for fatty breast, 24-26 kVp
Mo target, Rh filter - good for glandular breast, 27-31 kVp
Rh target, Rh filter - good for thicker breasts (> 7 cm)
Rh target, Mo filter - no, stop, this will do no good. Mo will filter out the Rh characteristic x-rays.
More coming soon
Computed Tomography (CT)
CT uses a rotating x-ray tube and a row of detectors to measure attenuation of the body at many angles. Those projections are then reconstructed into slides and 3D views to see structures inside the body.
Expand CT details
Hardware & scanning
Uses same sort of CT tube as for planar x-ray. Tube is opposite an arc scintillator detector (rare earth oxides) to convert x-rays to light photons, and photodiode to convert light to electrons. Electrons are integrated and digitized by ADCs.
Multi-row detectors typically (~96-320 rows in Z x 600-1000 channels in X). Collimation can change number of rows used.
Bowtie filter: compensates for different x-ray path length (reduce dose at periphery). Al + graphite.
Scanning parameters include current (mA), time (s), and voltage (kVp).
Current and time are typically given together as mAs.
Axial scanning: step and shoot. Table moves to position, stops, data acquired (tube/detector rotate around)
Helical scanning: continuous data acquisition as table moves.Pitch, table increment per rotation / beam width, can be adjusted.
Pitch < 1, overlap, pitch = 1, contiguous, pitch > 1, gaps.
Projection data reconstructed using FBP or iterative reconstruction for 3D volumes (similar techniques are also used for PET and SPECT).
Hounsfield unit: basic scale for CT images ("CT number"), based on attenuation coefficients, set such that water is 0 and air is -1000
$\text{HU} = 1000 \cdot \frac{\mu - \mu_w}{\mu_w - \mu_{air}}$
Image quality metrics
Spatial resolution: ability to resolve small objects. Tested with in-plane bar patterns, cross-plane by slice sensitivity profile or slice thickness. Depends on focal spot size, detector size, post-patient collimation, reconstruction kernel, reconstruction FOV (pixel size), and slice thickness.
Changing kernel will affect the MTF, and can be selected to highlight lung, bone, soft, details, etc.
Noise will increase with better resolution.
Low contrast detectability: depends on mAs, slice thickness, reconstruction kernel/algo.
Image noise: depends on mAs (linearly), kVp (not linearly), and reconstruction kernel/algo. Noise power spectrum (vs spatial frequency) provides magnitude and spatial correlation (texture) of the noise.
Artifacts
Cupping: reduction in HU at center due to beam hardening. Apply BH correction
Bone and metal can also cause streaks and shadows (reduced HU) from BH and photon starvation. Apply bone BH and/or metal artifact reduction correction, if available.
Aliasing: caused by sharp edges (metal, bone). Increase sampling rate or use smoothing kernel to reduce
Windmill: under-sampling in z-direction, increase sampling rate or use z-flying focal spot
Ring/bands: defects in detector cells, recalibrate or replace bad detector
Smudge: in center, from tube focal spot thermal drift or detector gain drift. Warm up or recalibrate
Tube Arching: debris inside tube insert, replace or season, can software correct also.
Motion: streaking and blurring, inadequate immobilization. Can use high pitch mode to reduce time.
Truncation: patient partly outsize FOV. Center as possible. Use largest FOV.
Dosimetry
CT dose depends on mAs, kVp, pitch.
mAs: dose increases with mAs linearly. Usually use 50 - 300 mAs. Can also be modulated depending on object thickness.
kVp: needs to be high enough to get through patient. Typically 100, 120, 140 kVp. Dose ∝ kVp2.6 depending on tube and filtering. Higher kVp allows lower absorbed dose for same detector exposure but also lower contrast (less photoelectric effect).
pitch: larger pitch, less dose (linearly)
CT Dose Index (CTDI): standard method of comparing doses for various scans. Given by CTDIw = (1/3) * radiationcenter + (2/3) * radiationperiphery for one slice in a phantom of length 100 mm (measured with 100 mm pencil ionization probe).
Use 16 cm head phantom and 32 cm body phantom.
CTDIvol = CTDIw / pitch for helical scans
Note: CTDI ≠ dose to patient! Corrections must be made based on patient size and what size phantom (32 cm or 16 cm) was used for the initial measurement.
See AAPM Report 204: Size-Specific Dose Estimates (SSDE) in Pediatric and Adult Body CT Examinations
Same CTDI will have higher SSDE for smaller patients.
Dose product length (mGy · cm): DPL = CTDIvol × scan (exposure) length
Effective dose: include tissue weighting factors: $D_E = \sum{W_T D_T}$ (mSv),
$D_E = \text{DLP} · k$ where $k$ is a factor depending on age and region scanned.
$k$ values in mSv / mGy / cm (AAPM Rpt No 96)
| Region | 1 year old | 10 year old | Adult |
| Head and Neck | 0.0085 | 0.0042 | 0.0031 |
| Head | 0.0067 | 0.004 | 0.0021 |
| Neck | 0.012 | 0.011 | 0.0059 |
| Chest | 0.026 | 0.018 | 0.014 |
| Abdomen/pelvis | 0.030 | 0.020 | 0.015 |
| Trunk | 0.028 | 0.019 | 0.015 |
Ultrasound
US (aka sonography) uses sound waves to create images of structures within the body.
Expand US details
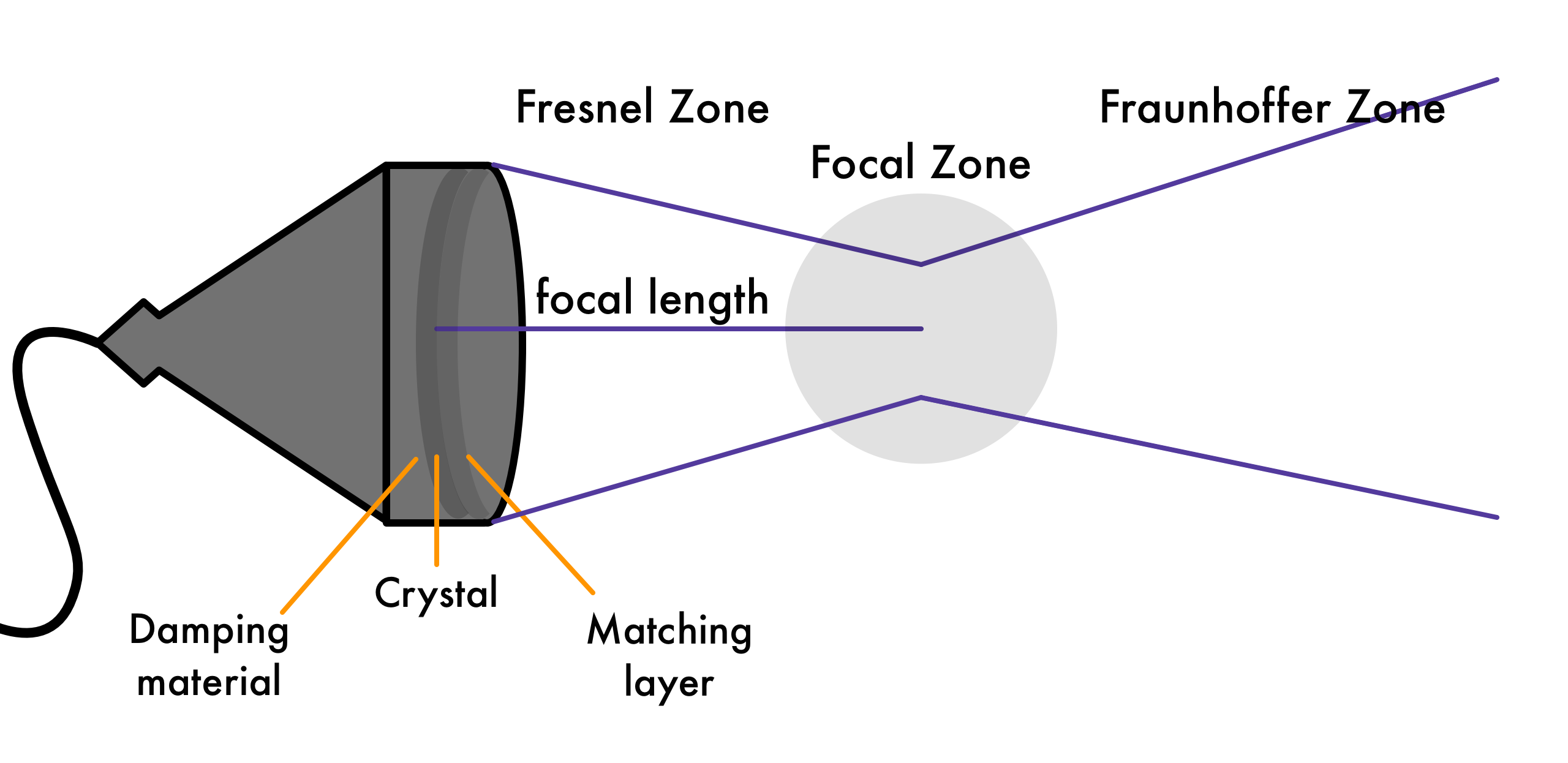
Transducer generates sound wave and also receives it. Resonant frequency of transducer is determined by thickness of piezoelectric crystal
$d = \lambda/2$
Acoustics
Diagnostic frequency in the 0.8-15 MHz range.
Speed of sound: $C = \lambda f$
$C = (K/\rho)^{1/2}$ where $\rho$ = density and $K$ = adiabatic bulk elastic modulus (resistance to deformation)
Impedance: $Z = \rho C = (K\rho)^{1/2}$ (kg/m2s = rayl)
Speed of sound in various tissue types
| Material | Speed m/s |
| Air | 330 |
| Water | 1430 |
| Fat | 1450 |
| Soft tissue | 1540 |
| Muscle | 1580 |
| Bone | 4080 |
Intensity: power/unit area = A2/area (W/m2)
Relative intensity: $\text{RI} = 10 \log(I_2/I_1) = 20 \log(A_2/A_1)$ (dB)
Half value layer (HVL) is $I_2/I_1 = 0.5 \rightarrow 10 \log(0.5) = -3$ dB
| Material | Acoustic Impedance (rayl) |
| Air | 0.0004 |
| Fat | 1.38 |
| Soft tissue | 1.54 |
| Bone | 7.8 |
Reflection fraction: $(Z_1 - Z_2)^2/(Z_1 + Z_2)^2$
Transmission = 1 - reflection
Obeys Snell's Law: $\sin(\theta_1)/\sin(\theta_2) = v_1/v_2$
Attenuation: $I = I_0 \exp(-\mu x)$
In dB: $\mu = \alpha f$ where $\alpha$ is material coefficient in dB/cm/MHz and $f$ is frequency in MHz.
$\alpha \approx 1$ db/cm/MHz for soft tissue.
Higher frequency → more attenuation.
Half-value-layer (HVL) = -3 dB → 10*log(0.5)
Tenth-value-layer (TVL) = -10 dB → 10*log(0.1)
| Material | Attenuation (dB/cm/MHz) |
| Water | 0.0022 |
| Fat | 0.5-1.8 |
| Soft tissue | 1 |
| Air | 12 |
| Bone | 20 |
Spatial Pulse Length (SPL) = $n\lambda$ = number of cycles * wavelength
Axial resolution = $n \lambda/2$ = SPL/2. $n$ is typically 3.
Axial resolution is better than lateral. High frequency → shorter spatial pulse width → better axial resolution but increased attenuation → less depth
Fresnel Zone (near field): Relatively constant pulse diameter (determined by transducer diameter), useful for imaging
Length of near field: $\text{L} = \frac{D^2}{4λ} = \frac{r^2}{\lambda}$
Fraunhofer zone (far field): Field diverges as $\theta = 70 λ / D \,\text{degrees}= 1.22 \lambda / d \,\text{radians}$
Higher frequency means less divergence, less blur.
Transducers
Piezoelectric crystal (voltage distorts crystal and creates pressure waves).
Thickness is half of wavelength: $d = \lambda/2$
Matching layer in front at $d = \lambda/4$ to prevent large reflections from acoustic impedance mismatches: $Z_t < Z_m < Z_p$
Damping block behind crystal to damp vibrations so it can receive signal
Listen to 20 cm depth is about t = d/v = 40 cm /1540 m/s = 260 μs
Can image, e.g., 225 lines at 17 frames/sec.
Pulse repetition frequency (PRF) = 1/travel time = 1/(d/v) = v/(2*depth). Typically 2-4 kHz.
Increase frame rate by decreasing depth, or reducing FOV
Doppler
Longer pulses, high quality factor: $Q = f/BW$
$\Delta f \propto \text{reflector speed}$
$f_{shift} = 2 \cdot (v/c) \cdot f_0 \cdot \cos(\theta)$ where $v$ is velocity of reflector, $c$ is speed of sound, $f_0$ is transducer frequency, and $\theta$ is angle of velocity wrt sound wave.
US Artifacts
Expand artifacts details
This RadioGraphics paper is a good resource with several examples of artifacts with illustrations and images.
- acoustic enhancement - increased echoes deep to structures that transmit sound exceptionally well.
- acoustic shadowing - signal void behind structures that strongly absorb or reflect ultrasonic waves.
- aliasing artifact - occurs in Doppler modalities which use intermittent sampling with an insufficient sampling rate. Results in an inability to record direction and velocity accurately.
- anisotropy - fibers directing sound away from transducer and look hypoechoic. occurs with decreased insonating angle.
- beam width artifact - reflective object located beyond the widened ultrasound beam, after the focal zone, creates false detectable echoes that are displayed as overlapping the structure of interest.
- blooming artifact - color signal indicating blood flow extends beyond its true boundaries, spreading into adjacent regions with no actual flow. From lower spatial resolution of the Doppler vs grayscale image.
- comet tail artifact - seen when small calcific / crystalline / highly reflective objects are interrogated and is perhaps be a special form of reverberation artifact.
- electrical interference artifact - moving arc-like bands appear in image, when US machine is too close to the unshielded electrical equipment
- transducer-related artifact - from malfunctioning transducer or cable
- mirror image artifact - highly reflective surface (e.g. diaphram) in the path of the primary beam and another objects reflects back to it and then back to transducer. Signal time is increased and a deeper mirror version of the image appears also.
- multipath artifact - primary beam reflects off anatomy at an angle, resulting in a portion of the beam returning to the transducer, while another portion takes a longer duration as it reflects a second structure and appears deeper
- reverberation artifact - occurs when signal reflects back and forth between two strong parallel reflectors and appears deeper
- refraction artifact - pulse strikes an interface at a non-perpendicular angle. The difference in propagation speeds between the two tissues can cause refraction to occur. If wave strikes a reflector, location might appear incorrect
- ring down artifact -Resonance artifact, looks similar to comet artifact. Pulse encounters a "horn" or "bugle" shaped fluid collection that is trapped between an inverted tetrahedron of 4 bubbles (3 on top and 1 nestled deep to them) The trapped fluid resonates, emitting a continuous signal back to the transducer of distinct frequencies. "Beats" between these frequencies produce the variable appearance of the ring down. Displays as ling of bands posterior to gas.
- side lobe artifact - side lobes reflect sound from strong reflector that is outside of the central beam, and where the echoes are displayed as if they originated from within the central beam. Echogenic, linear or curvilinear artifacts.
- speckle artifact - scattering of waves from the surface of small structures within a certain tissue. Produces a textured appearance.
- speed displacement artifact - area of focal discontinuity and displacement of an echo deeper than that its actual position in an imaged structure. If speed of sound in object is different than expected (e.g. 1540 m/s).
- twinkling artifact - intrinsic machine noise seen with color Doppler ultrasound. A focus of alternating colors on Doppler signal behind a reflective object
Magnetic Resonance
MR uses a strong magnetic field and radiofrequency waves to generate anatomical and functional images of the inside the body.
Expand MR details
Units: Tesla (SI), 1 T = 10,000 gauss (historical)
Magnetic fields can be induced by moving charges (Maxwell's equations). Direction of induced magnetic fields are determined by the right hand rule: fingers along direction of moving positive charge, thumb then points in direction of the magnetic field.
Nuclear Magnetic characteristics of elements
Elements must have spin and charge --> Odd # of protons or neutrons
If Nn+Np is odd, nucleus will have 1/2 integer spin
If Nn and Np are odd, nucleus will have integer spin
If Nn and Np are even, nucleus will have zero spin
Common MR active nuclei:
1H, 13C, 19F, 23Na, 31P
Most signal comes from 1H: largest magnetic moment along with highest abundance (of atoms with MR signal).
Magnetic moment: $\mu_z = \pm \frac{\gamma h}{4 \pi} $
Magnetism in materials:
Susceptibility: how magnetized a material becomes in a magnetic field.
Diamagnetic: slight negative susceptibility, oppose the applied field because of paired electrons in orbitals. Calcium, water, organics (carbon and hydrogens)
Paramagnetic: slight positive susceptibility, enhance applied field because of unpaired electrons. O2, some blood parts, gadolinium contrast agents.
Ferromagnetic: large positive susceptibility, substantially augment applied field, have self-magnetism. Iron, cobalt, nickel. Will distort signals.
MR fields
The performance of magnets in medical imaging depend on field strength, temporal stability, and field homogeneity. Electromagnetic core wires must be superconductive to achieve the >= 1 T used in clinical imaging. Superconductivity, no resistance to electric current, is achieved through the use of special metals held at extremely low temperatures (a few Kelvin). Cooling is typically done with continually replenished liquid helium (~4K).
In addition to the main magnetic field of an MR machine (1.5 - 3 T clinically, 4 -7 T research), B0, there are also shim coils to aid in generating a homogeneous field, gradient coils to produce linear variations in field strength (for localization), and RF coils for sending an receiving signals that generate contrast in the tissues to form images.
Fringe field limit: 0.5 mT = 5 G.
The gradient field is generated from two or more coils each creating magnetic field variations that combined induce a slight linear change in the main B0 on the order of 0.005 T/m. [more on localization later]
Excess spins based on Boltzmann statistics - difference in energy between states
$N_{anti}/N_{parallel} = \text{exp}(\frac{\Delta E}{kT}) = \text{exp}(\frac{\gamma h B_0}{2 \pi k T}) \approx 1 + \frac{\gamma h B_0}{2 \pi k T}$
Each tissue voxel contains ~1021 protons → ~3x1015 excess spins in low energy state per voxel.
Larmor equation: $$\omega = \gamma B_0$$
Angular momentum of proton spin is determined by the nucleus-specific gyromagnetic ratio $\gamma$ and the main magnetic field $B_0$
For hydrogen (1H), $\gamma$ = 42.57 MHz/Tesla
Bloch equation: $\frac{dM}{dt} = \gamma M x B$
$M_z$ is magnetization along $B_0$ field. $M_{xy}$ is magnetization in the perpendicular plane. RF pulses can be used to flip spins to different combinations.
The flip angle depends directly on the power of the RF field and the length of the pulse.
For the same power, a pulse that lasts twice as long will flip 2x the angle.
Apply 90 degree RF flip → $\theta = \gamma B_1 t = \pi / 2 $ → need field of ~ 0.1 G for 1 ms
Maximizes $M_{xy}$ and minimizes $M_z$
Precession in transverse plane (only) generates the MR signal (induces current in receiving coil).
$M_z$ exponentially relaxes over time to return to net magnetization along $B_0$ field.
$$M_z=M_0 (1-\text{exp}(-t/T1))$$
Different tissues relax at different times (T1s) and that is where contrast comes from. (Microscopic energy interactions between molecules.)
Spin-lattice relaxation, T1, is time to return to 63% of $M_0$. Fully recovered in 5*T1. T1 depends on field strength. Fat relaxes faster than water (smaller T1). Typically 0.1 to 1 second.
Long T1 is dark, short T1 is bright. Useful for anatomy.
air, calcium, cortical bone, fast blood : fluid, muscle, organs, proteinous tissue : blood, fat, bone marrow, contrast agents
(similar ordering as O/C ratio is tissues (light to dark): Fat < liver < organs < WM < muscle < GM < CSF)
Spin-spin relaxation
After 90 degree RF flip, $M_z$ is zero, $M_{xy}$ is maximized and rotates at Larmor frequency. Coherence in xy plane is gradually lost. Decay of signal is detected by receive coil.
Loss of MR signals is called Free Induction Decay (FID).
Demo of xy plan coherence loss (WORK IN PROGRESS)
Plot $\Sigma M_{xy}$T2 is the decay time (half-life) of the transverse magnetization. It occurs because of magnetic field inhomogeneities (each spin sees slightly different $B_0$), from external field and structure of material. It is independing of the main field strength. T2 fat < T2 water
$M_{xy} = M_0 \text{exp}(-t/T2)$
short T2 is dark, long T2 is bright. Useful for pathology (edema).
air, calcium, cortical bone, fast blood : fat, ligaments, cartilage, mucles, bound water : fluid, CSF, pathology
T2 decay from tissue effects, T2* includes external effects, and is always faster.
T1 > T2 > T2*
High tumbling rate (non-viscous), high T1 and high T2 Low tumbling rate (solids), high T1 and low T2
Measure T1 using two sets of 90° pulses. Estimate from recovery curve.
T1-weighted images use a short TR and short TE (emphasize differences in T1 and deemphasize T2 differences).
T2-weighted images use a long TR and a long TE
Slice selection and localization
slice selection details
Slices and position information in MR is set by adding very subtle gradients to the magnetic field. By adding/subtracting from the magentic field, each region has a slightly different resonant (Larmor) frequency, which can be detected by the receiving coil.
E.g., instead of a single 64 MHz, frequency might range from 64 to 64.009 MHz.
The peak amplitude of the gradient determines the slope (usually 1-50 mT/m).
The slew rate is the time to achieve peak amplitude (5-250 mT/m/ms) - limited by eddy currents.
Slice selection is often along the z-direction (along the $B_0$ field). Applied at the time of the inital RF pulse to excite slice corresponding to exact RF frequency. Thickness of slice depends on RF pulse bandwidth and slope of the gradient.
Narrow bandwidth → small Δz
High gradient → small Δz
Longer pulse → sharper slice profile
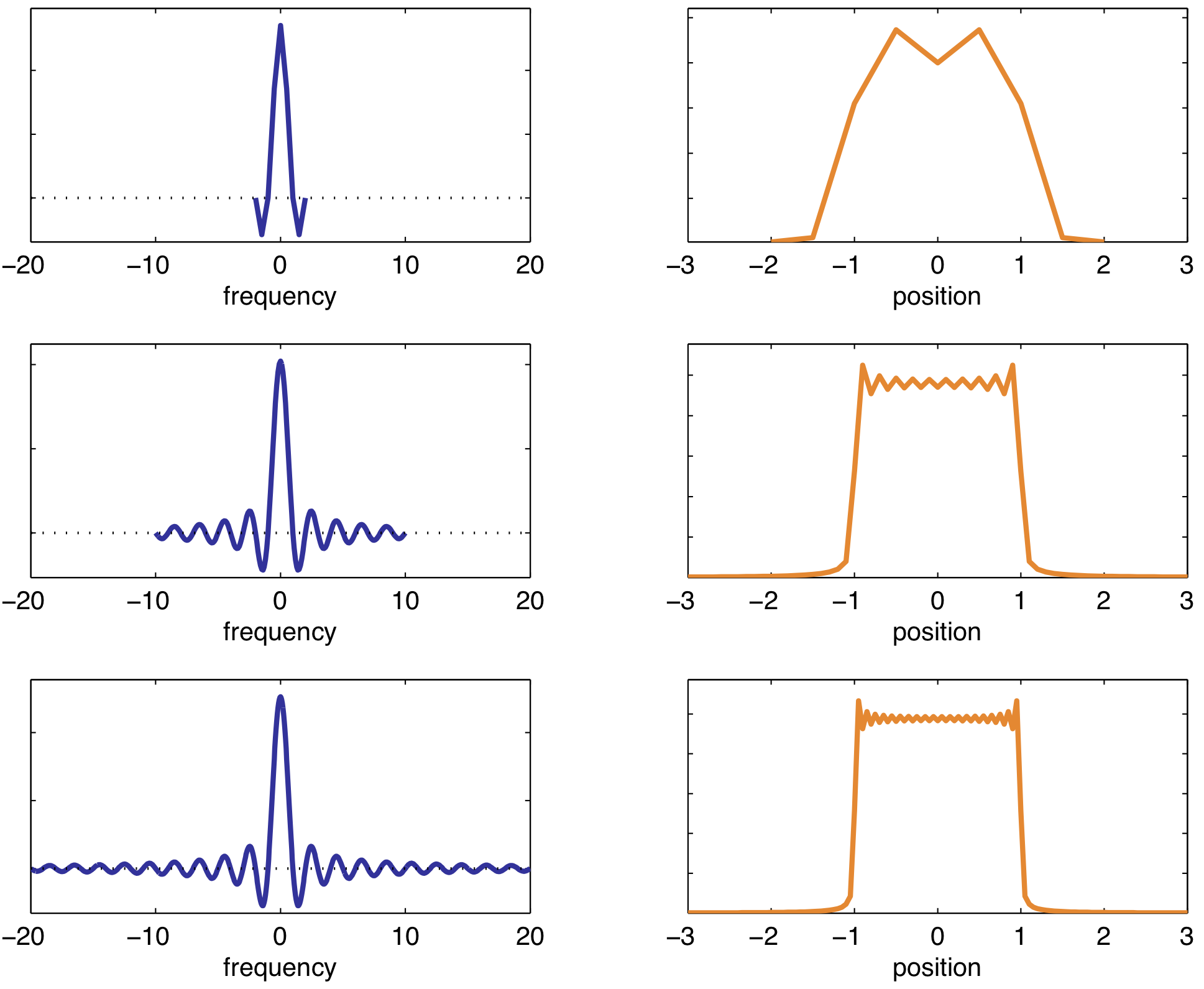
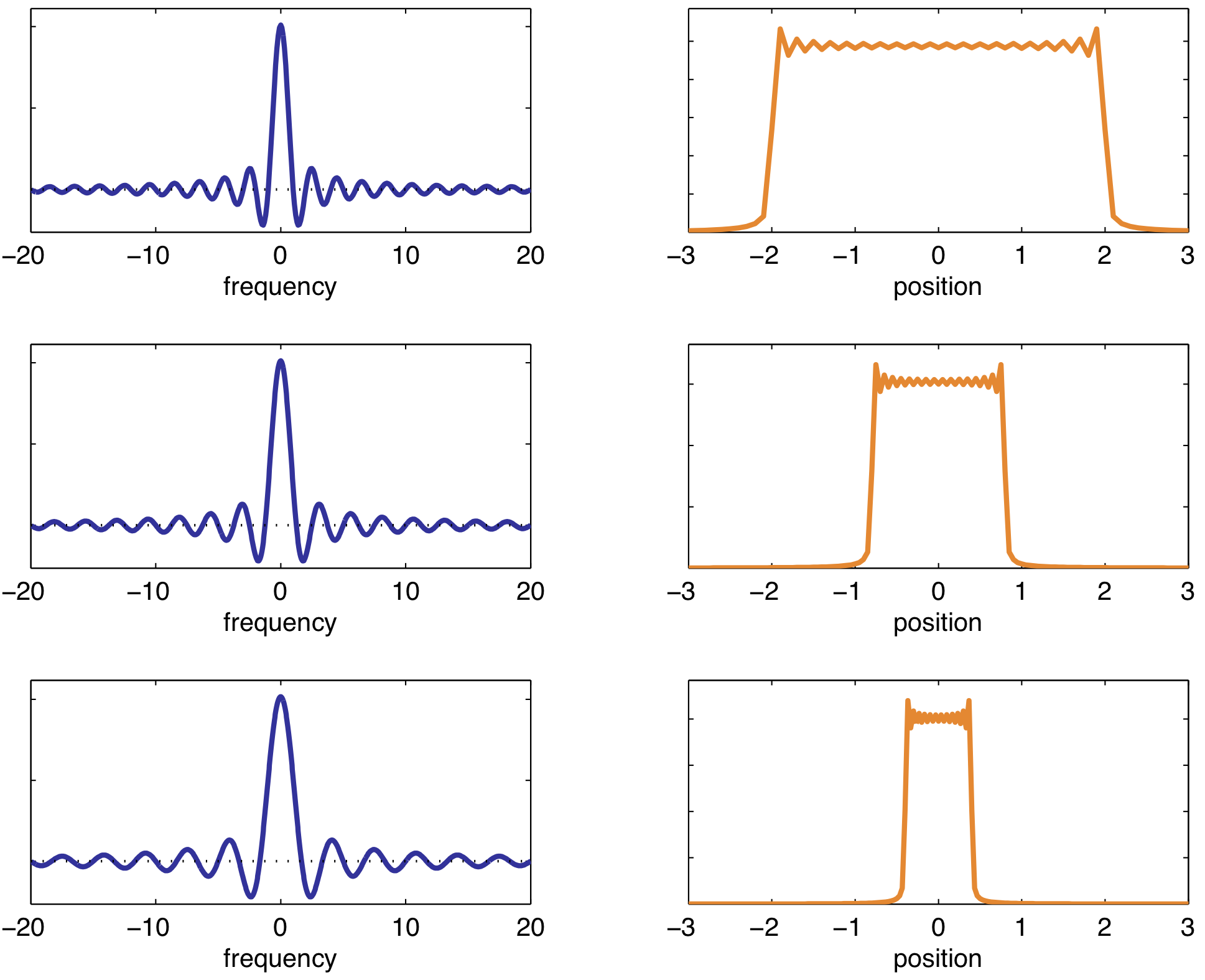
A sinc pulse is used to excite a rectangular slices (Fourier transform of each other). Longer RF pulse → sharper slice profile (more "lobes" included in the sinc pulse).
Bandwidth also determines the SNR: $\text{SNR} \propto 1/\text{BW}$
GX and GY gradients used phase and frequency encoding
Phase: precessional frequency is altered by a y-gradient. When Gy is off, all parts have same frequency again but are now phase shifted.
Frequency: applied in x-direction at signal readout time. Frequency is altered with Gx, and differences are used to determine locations. [IMAGE - slide 59 lec 17-18]
Precision of localization (frequency and phase in k-space) depends on precision of $B_{tot}(\vec{r}) = B_0(\vec{r}) + G(\vec{r})r$
Sequences in MR
Sequences details
Spin-Echo sequence
Starts with 90° pulse into xy plane, resulting in FID signal decaying at T2*
180° pulse after TE/2 inverts the spins and reestablishes phase coherence, $M_{xy}$ is maximized at TE
Sequence is repeated, starting with another 90° pulse, every TR (repetition time).
True T2 value is estimated from the resulting curve.
T1 - spin-lattice relaxation, net magnetization relaxing back to equilibrium, $M_0$, after excitation:
$M_z = M_o (1-\text{exp}(-t/T1))$
Higher $B_0$, longer T1
| Field Strength (T) | Tissue | T1 (ms) | T2 (ms) |
| 1.5 | WM | 510 | 67 |
| GM | 760 | 77 | |
| Blood | 1441 | 290 | |
| CSF | 2650 | 280 | |
| 3.0 | WM | 1080 | 70 |
| GM | 1820 | 100 | |
| Blood | 1932 | 275 | |
| CSF | 3817 | 1442 |
| Field Strength (T) | Time (ms) | WM | GM | Blood | CSF |
| 1.5 | T1 | 510 | 760 | 1441 | 2650 |
| T2 | 67 | 77 | 290 | 280 | |
| 3.0 | T1 | 1080 | 1820 | 1932 | 3817 |
| T2 | 70 | 100 | 275 | 1442 |
|
Tissue |
T1 (ms) @ 1.5 T |
T2 (ms) |
|
CSF |
>4000 |
>2000 |
|
WM |
884 |
92 |
|
GM |
1124 |
101 |
|
Fat |
288 |
84 |
|
Muscle |
1130 |
47 |
|
Liver |
586 |
43 |
|
Kidney |
966 |
58 |
|
Spleen |
1057 |
62 |
- T2 - Spin-spin relaxations, net magnetization in XY (transverse) plane is gradually lost: $M_{xy} = M_0 \text{exp}(-t/T2)$
- T2* includes additional dephasing from magnetic susceptibility and other outside effects: 1/T2* = 1/T2 + Δω
- Gradient Echo sequence: 90° RF pulse (create transverse magnetization in one slice), Gy phase encoding, Gx frequency encoding, read signal at time TE, repeat with time TR
- Can spoil the signal to ensure $M_T = 0$ by end of TR
- Shorter TR, T1-weighted; Longer TR, proton density (PD) weighted.
- Partial saturation recovery, short TE, PD-weighted; Long TE, T2*-weighted.
- Flip angle often less than 90° - higher signal early, but tapers off more quickly - useful for multiple pulse preps
- Ernst angle: flip angle that maximizes signal for a given TR: $\theta_E = arccos(e^{-TR/T1})$
- Spin-Echo signal (90° then 180°): $S = M_0(1-e^{-TR/T1})e^{-TE/T2}$
- Values for 1.5 T, higher fields need longer TR
- Short TR, T1-weighted; Long TR, PD weighted
- Short TE, PD-weighted; Long TE, T2-weighted.
- PD-weighted: TR ~ 2000 - 5000 ms ~ 3*T1 and TE ~ 5-20 ms << T2
- T1-weighted: TR ~ 300 - 500 ms ~ T1 and TE ~ 5-20 ms << T2
- T2-weighted: TR ~ 2000 - 5000 ms ~ 3*T1 and TE ~ 80-120 ms ~ T2
Signal, $S$ depends on proton density, $H$, the mean relaxation times $T_1$ and $T_2$ of the tissues, and the repetition, $TR$ and echo, $TE$ times.
$$S = k \cdot H \cdot (1- e^{-TR/T1}) \cdot e^{-TE/T2}$$
Short TE, and last term goes to one (T2 has little effect)
Long TR and middle term goes to one (T1 has little effect)
|
|
Short TR |
Long TR |
|
Short TE |
T1 weighted, Brightness: Fat > liver > organs > WM > muscle > GM > CSF |
PD weighted Brightness: WM < GM ~ CSF (lower contrast) |
|
Long TE |
N/A |
T2 weighted, Brightness: muscle < organs < fat < WM < GM < CSF |
- Encoding kx ~ 2-10 ms, ky ~ 1s - 5 min → total time = TR * #ky lines ~ 3 s * 256 ~ 15 minutes
- Echo-Planar: speed up ky sampling - read entire k-space in a single RF preparation.
- Needed for functional imaging
- Dynamic Susceptibility Contrast (DCS) - Gd bolus, paramagnetic
- T2* weighting to highlight Gd3+ - contrast shortens the T2*, increased R2* linearly with concentration
- Signal is not as strong
- Blood oxygen level dependence (BOLD) for fMRI
- Suscptibility decreases, relaxtivity decreases, T2* increases, MR signal increases
- Look for differences in signal between rest and activated states (usually 2-5%)
- Determine areas of significance with Z-score: $Z = (m_a - m_r) / SD$
- Diffusion weighting
- Moving spins along gradient experience phase shifts
- Sensitive to motion - need fast encoding - EPI
- Can detect edema (restricted diffusion, high signal)
- Tractography with diffusion tensor imaging (DTI)
Artifacts
Artifacts details
- Aliasing
- k-space (digital) effect
- From finite, digital sampling and fourier transforms
- Additional sampling might be needed (but adds time)
- Wrapping of images - usually in phase direction - FOV divded into 360° and anything outside will be given other angles of equivalent values (e.g. 450 mismapped to 90°)
- Truncation
- k-space (digital) effect - have to cut off frequencies at some point
- Effect seen can depend on matrix size, adds a ripple pattern
- RF interference
- External sources of narrow RF seen as bands along phase direction at specific frequencies
- Broadband interference adds speckle/noise (poor SNR)
- Rawdata spike
- Hatch-like pattern from bright spots in k-space
- Slice crosstalk
- Residual magnetization from previous slice excitation (boundaries aren't perfectly sharp)
- Acquire slices interleaved so as not to excite adjacent slices in a row
- Flow void
- Excited spins flow out of imaged slice, resulting in lower signal
- Signal might appear on next slice
- Faster motion more affected than slower motion
- Flow enhancement
- Excited spins flowing in from previous slice
- Ghosting (motion)
- static + velocity + acceleration + jerk + ....
- Motion along gradient leads to phase shift error
- Seen in phase encoding direction because it takes much longer to acquire (standard cartesian encoding)
- Radial sampling can mitigate erros
- Ghosting (pulsation)
- Artifacts often have regular spacing corresponding to interaction of periodic phase encoding and periodic pulsation
Localization precision: depends on precision of $B_{tot}(\vec{r}) = B_0(\vec{r}) + G(\vec{r})r$
Chemical shift: electron distributions create local magnetic fields that can shift Larmor frequency slightly depending on chemical structure. Can be used for NMR spectroscopy, or may create image artifacts.
Difference in frequency between fat and water is ~ 200 Hz @ 1.5 T and 440 Hz @ 3.0 T. Diff of ~3.5 ppm.
Slightly different locations will be exicted along gradient for fat vs water - slice to slice or in freq. encoding direction
Magnetic susceptibility: $B_0 \propto (1+\chi) H_0$ - experienced field strength $B_0$ vs field of $H_0$.
Tissues have different χ, and metalics can affect as well.
Eddy currents - Faraday's law of induction
Can distort gradient pulse shapes, and result in shearing, stretching and translation.
Modality comparison, image features and artifacts
Comparison of image features, image quality, artifacts, etc., between the main diagnostic imaging modalities: planar x-rays, CT, US, MRI.
Expand modality comparison details
Detective Quantum Efficiency (DQE): Characterizes the frequency dependent SNR performance of an imaging system.
$\text{DQE} = (\text{SNR}_{out}/\text{SNR}_{in})^2$
Signal-to-Noise Ratio (SNR): signal relative to the noise in an image:
$$\text{SNR} = \frac{\mu_{sig}}{\sigma_{bkgd}} = \frac{\mu_{sig}}{\sqrt{\sigma_{bkgd}^2 + \sigma_{sig}^2}} = \frac{\sum_i{(x_i - \bar{x}_{bg})}}{\sigma_{bkgd}}$$’
Contrast-to-Noise Ratio (CNR): signal-background relative to the noise in an image: $\text{CNR} = \frac{(\mu_{sig} - \mu_{bkgd})}{\sigma_{bkgd}}$ or $\text{CNR} = \frac{(\mu_{sig} - \mu_{bkgd}) \,}{\sqrt{\sigma_{bkgd}^2 + \sigma_{sig}^2}}$
Rose criterion: SNR limit for likely detection of an object. Usually around SNR > 3-5
Noise power spectrum: visualization of the frequency dependence of noise (how much structure is has).
White noise has no frequency dependence
$\int{\text{NPS}} = \sigma^2$ (variance)
For MRI, SNR proportional to voxel volume, and square root of number of averages and phase steps. Can also increase signal by decreasing TE and increasing TR
Higher SNR takes more time.
Methods of quality control and quality assurance
Basic quality control/assurance tests and dose considerations
In progress...
Expand QC details
Typical radiation doses:
| Exam Type | reference dose level (mSv or mGy) |
| Extremity | 0.001 mSv |
| Mammography | 0.02 to 0.1 |
| Adult PA check | 0.15 mGy |
| Adult PA abdomen | 3.4 mGy |
| Upper GI fluoroscopy | 56 mGy/min |
| Adult CT head | 75 mGy CTDI |
| Adult CT abdomen | 25 mGy CTDI |
| Pediatric CT abdomen | 20 mGy CTDI |
| Pyelogram (kidneys) | 2.5 |
| Angio | 7.5-57 |
| Coronary | 4.5 - 16 |
| FDG | 14 |
| SPECT Tc99m | 2-7 |
| SPECT 201Tl (cardiac) | 12 |
| SPECT 67Ga (tumor/infection) | 19 |