Radiation Biology
- Physics and chemistry of radiation interactions with matter
- Molecular and cellular radiobiology
- Tumor radiotherapy
- Normal tissue response to radiotherapy
- Time dose fractionation
- Radiobiological basis of radiation protection
- Radiation accidents and environmental radiation exposure
- Diagnosis and medical management of radiation syndromes
- Deterministic effects
- Stochastic Effects
- Radiation carcinogenesis
- Heritable radiation effects
- Effects on the developing embryo
Physics and chemistry of radiation interactions with matter
Cell / chromosome damage and repair
X- and gamma-rays are indirectly ionizing; they produce fast recoil electrons.
Neutrons are indirectly ionizing; they produce recoil protons, alphas, and nuclear fragments
Energy and wavelength: E = hf | λÅ = 12.4/E(keV) → 0.1Å = 124 keV
In Compton scattering range (1-10 MeV), soft tissue, muscle, and bone have similar absorbed dose (not so in photoelectric range - 40 keV).
Radiation effects on cellular level:
Direct effects - recoil electron ionized target - DNA strand damage - cell death, mutation, inability to replicate, or reparation
Indirect effects - recoil electron creates free radicals (atom with unpaired valence electron) - hydroxyl (•OH), super oxide (O2), hydrogen peroxide (H2O2)
Very chemically reactive (ion radicals live 10^-10 s, then become uncharged radicals)
# ion species proportional to dose
Direct causes 1/3, indirect causes 2/3 of damage (x-rays).
10-18 energy absorbed
10-16 physics
10-12 free ion radicals
10-9 free radical (OH•)
10-5 chemistry (DNA radical lifetime)
hrs-months for cell death
hrs-years for gross biological effects
H2O + gamma/xray → H2O+ + e-
H2O+ + H2O → H3O+ + OH• (hydroxyl radical, highly reactive)
Neutrons - recoil protons or spallation (split, typically into alpha particles)
Heavy ions and neutrons have higher proportion of direct ionization
Radical scavenging – compound that reacts with certain free radicals, reduce biological effects
Sensitizing chemicals also exist, to enhance effect of free radicals.
Linear energy transfer (LET) effects:
Low LET: 70% indirect, 30% direct. Mostly OH and e-aq. Diffusion controlled.
High LET: radicals decrease and molecules increase
LET is typically in keV/μm.
Not homogenous
- Spurs < 100 eV, ~ 4nm, ~ 3 ion pairs (95% for x/γ-rays)
- Short tracks
- Blob 100-500 eV, ~ 7nm, 12 ion pairs
- Branch tracks > 5000 eV
"Locally multiply damaged site", now "clustered lesion" - ~20 base pairs
Spurs/blobs around size of DNA double helix
DNA is primary target:
- Sensitive if are DNA repair deficient or have drugs to inhibit repair
- Multiple interactions of •OH with bases and sugar of DNA
- Breaks – SSB (alkaline), DSB (neutral), base changes/loss, cross links DNA-DNA and DNA-protein – usually linear with dose
DBSs most relevant for cell killing and chromosomal aberrations at cell division. Occur linearly with dose.
Oxygen Effect/Enhancement Ratio (OER) = Dose hypoxic region / Dose oxygenated region, ~ 3 at high doses (lower @ lower dose). Extra dose needed for same killing effect.
Time for effects (seconds)
Oxygen reactions:
R• +O2 →RO2• (highly toxic)
H• +O2 →HO2•
HO2•+HO2•=H2O2 (highly toxic)+O2
where R represents an organic molecule
DNA damage
Types, and damage by 1-2 Gy:
- single strand breaks (SSBs), ~ 1000
- double strand breaks (DSBs), ~ 40
- base damage, >1000
- protein-DNA cross links
- protein-protein cross-links
Many mechanisms for DNA repair, so small % of damage cause cell death. DSBs are most lethal, most likely to lead to carcinogenesis.
Damage classification
- Sublethal damage
- under normal circumstances, can be repaired (~hours)
- additional SLD can impair repair and lead to lethal damage
- Potentially lethal damage
- can be modified by post-irradiation environmental conditions
- higher survival if post-irradiation conditions are suboptimal for growth
- No mitosis with damaged chromosomes
- Lethal damage
- irreversible, irreparable, cell death
- Somatic effects
- Limited to individual, not later generations (genetic)
- Deterministic effects
- Threshold dose for effects. Severity increases with dose
- Radiation syndrome
- Threshold dose for effects. Severity increases with dose
- Stochastic effects
- No threshold dose, probabilistic. Probability, not severity. increases with dose.
- Cancer
- No threshold dose, probabilistic. Probability, not severity. increases with dose.
Measurements of damage
Measure base damage with 3H from thymine, phosphate release, fluorescent labeling, immune probs, HPLC, enzyme sensitivity
Measure strand breaks with sucrose gradients, alkali unwinding… , filter elution, gel electrophoresis, comet assay, Foci of repair proteins
Gel electrophoresis: use alternating current to move strands based on size (megabase-pair range)
PFGE (pulsed field GE) is most widely used method to detect induction and repair of DSBs. More displacement with more dose (smaller fragments)
Comet assay: single cells in agarose gel, electrophorese, DNA in tail if damaged. If not damaged, compact and spherical, otherwise spreads.
Detects SSBs under alkaline conditions, and DSBs with neutral pH.
Nuclear Foci assay: Signaling and repair protein foci induced from DNA damage. Bind with an antibody and detect with fluorescent antibodies.
Proteins: γH2AX and c53BP1
Mechanisms of DNA repair
Base excision repair (BER): Mutation removed, replaced with correct base. May lead to increased mutation.
Nucleotide excision repair (NER): Removes bulky adducts (e.g., pyrimidine dimers). Incisions bracketing lesion (24-32 nucleotides in length), removal, repair synthesis, ligation.
Can be global or only for actively transcribed genes.
Mutations in NER can lead to xeroderma pigmentosum (UV hypersensitivity).
SSBs can often use opposite strand as template for repair.
DNA double-strand break repair:
Homologous recombination repair (HRR) - needs undamaged DNA strand (chromatid/chromosome) as a template, error-free.
Occurs mostly in late S/G2 phase (have a copy).
Dominant in lower eukaryotes (yeast).
Inactivations of HRR genes results in radiosensitivity, genomic instability and decreased proliferation.
Non-homologous end-joining (NHEJ) - mediates end-to-end joining (repair proteins clean up broken ends, then ligate). Error-prone (generates antibody diversity) and creates aberrations, immune deficiency, radiation sensitivity.
Occurs in G1/early S phase (no sister template available)
Crosslink Repair: under investigation. combination of NER and HRR pathways is needed to repair crosslinks.
Chromatin with actively transcribed genes is more susceptible to crosslinks.
Mismatch Repair (MMR) - removes base-base and small insertion mismatches occurring during replication. Excise incorrect nucleotides, re-synthesized, and ligation of DNA tract.
Mutations lead to micro-satellite instability (insertions/deletions) and cancer, esp. hereditary nonpolyposis colon cancer (HNPCC).
Unrepaired or misrepaired damage leads to mutation (possibly cancer), and'/or chromosome damage (often cell death).
Cell division
Short DNA strand ~ 2 nm
Chromosome ~ 1400 nm
Cell division (mitosis): Prophase - thickening of chromatin, chromosomes as light coils then condensing. Metaphase - chromosomes to center of cell, spindle forms linking cell poles, centromeres divide. Anaphase - movement of chromosomes towards poles, arms trail. Telophase - chromosomes at poles uncoil, membrane reappears.
Telomeres: caps on chromosomes that are gradually lost during cell divisions. Eventually cells undergo senescence.
Telomerase enzyme can add to ends and avoid degradation from division (e.g., cancer cells and stem cells).
Chromosome damage has been studied in plants because they have fewer and larger chromosomes.
DBSs can restitute (recombine, behave normally), fail to rejoin (aberration/deletion at mitosis), rejoin other broken ends (distortions)
Types of aberrations from radiation
Aberrations happen heterogeneously across chromosomes (e.g. 8 is sensitive to exchanges)
Chromosome aberrations - irradiation early in interphase (G1), before duplication. Breaks are replicated
Chromatic aberrations - irradiation in late interphase (S/G2), after duplication, to a single arm.
Dicentric: lethal, chromosomal. Interchange between two chromosomes, both broken and join together + fragments (no centromere).
Ring: lethal, chromosomal. Break in each arm of a chromatid. Ends join and form ring + fragment
Anaphase bridge: lethal, chromatic. Breaks in late cell cycle (G2), after replication. Break in both chromatics and rejoin incorrectly.Two centromeres, pulled to opposite poles.
Symmetric translocation: non-lethal. Break in two pre-replication (G1) chromosomes, ends exchanged. Can see with fluorescent in situ hybridization (FISH), or "chromosome painting". Can activate onogenes (some lymphomas and leukemias).
Small interstitial deletion: non-lethal. Two breaks in same arm, small fragment lost. Carcinogenic if tumor suppressor gene is lost.
Lymphocytes often used as biomarkers for radiation exposure -- frequency of dicentrics and rings corresponds to total body dose.
Can detect exposures as low as 0.25 Gy (in cases of anomalous badge readings or accidents)
Dicentrics and rings decline with time (cells die and can't replicate), but translocations and small deletions can persist (able to replicate with the error).
Cell Survival curves
Relationship between dose and proportion of cells that survive (proliferate and/or function).
Reproductive integrity remains if a colony can be formed (multiple reproductions occur), i.e., it is clonogenic
Easy to verify reproductive integrity by growing colonies, visible by naked eye
Most cells die a mitotic death, some die through apoptosis (programmed cell death).
Apoptosis - Chromatin condensation and DNA fragments, usually p53-dependent, Bcl-2 suppresses. Hemopoietic and lymphoid cells pathway. Laddering in gel electrophoresis. Survival is exponential function of dose, little dose-rate effect. Contributes to alpha. If dominant mode of cell death, then radiosensitive.
Mitotic - Reproductive death, most common, die when dividing (first or later division). Contributes to alpha and beta.
Autophagy - Self digestive, lysosomal degradation of protein and organelles (programmed type II death). Possible cancer therapy target
Necrosis - Loss of cellular membrane activity. Occurs after high radiation doses.
Senescence - Stress or age leads to silencing of genes to promote transition from G1 to S phase (no dividing). Regulated by p53 and Rb proteins. Still metabolically active.
Need 100 Gy to destroy cell function, but only 2 Gy to stop proliferation.
Cell cultures: known number of cells placed in two culture vessels. One is irradiated, one is control. Number of colonies growing in control / total cells plated = plating efficiency (usually 50-90%). The number of cells placed in irradiated dish is adjusted for plating efficiency (and usually start with many more). The number of colonies that grow relative to expected (cells placed * efficiency) is the surviving fraction. Obtain numbers for a range of doses to get survival vs dose curve.
Cell lines: cells cultured that did not die out after a few weeks (typical) and are considered "immortal" established cell lines.
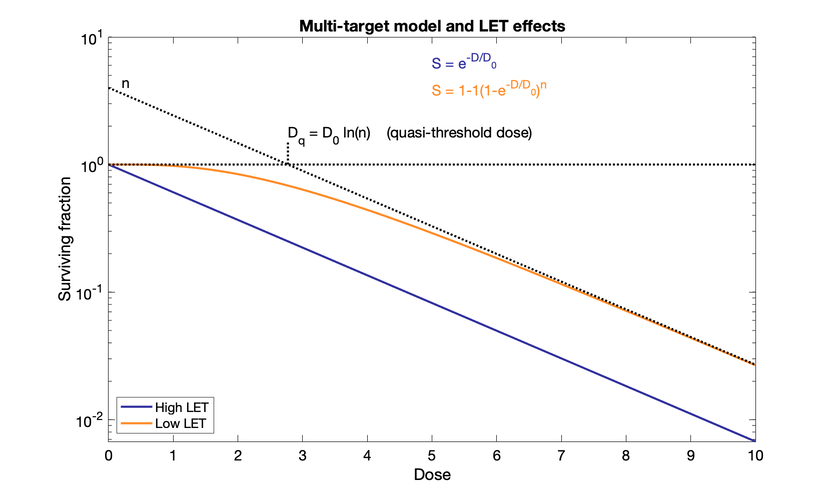
Multi-target model (older)
Reciprocal of initial slope $D_1$ from single event killing and reciprocal of final slope $D_0$ from multiple-event killing. They are dose required to reduce the fraction of surviving cells to 37% (1/e) of its previous value.
Quantity $n$ or $D_q$ to represent size of shoulder. $D_q$ is quasi-threshold dose: dose where the straight part of curve, extrapolated backward, crosses a surviving fraction of unity. $n$ is where extrapolated line crosses y-axis. Large $n$ → broad shoulder. "Quasi" because there is no actual threshold dose with no effect.
Ds are the dose required to reduce fraction of surviving cells to 37% of previous value.
Curve straightens at higher doses.
$\ln(n) = D_q / D_0$
$D_0$ for cultured cells is around 1 to 2 Gy. Hypersensitive cells are around 0.5 Gy (from patients with cancer-prone syndromes)
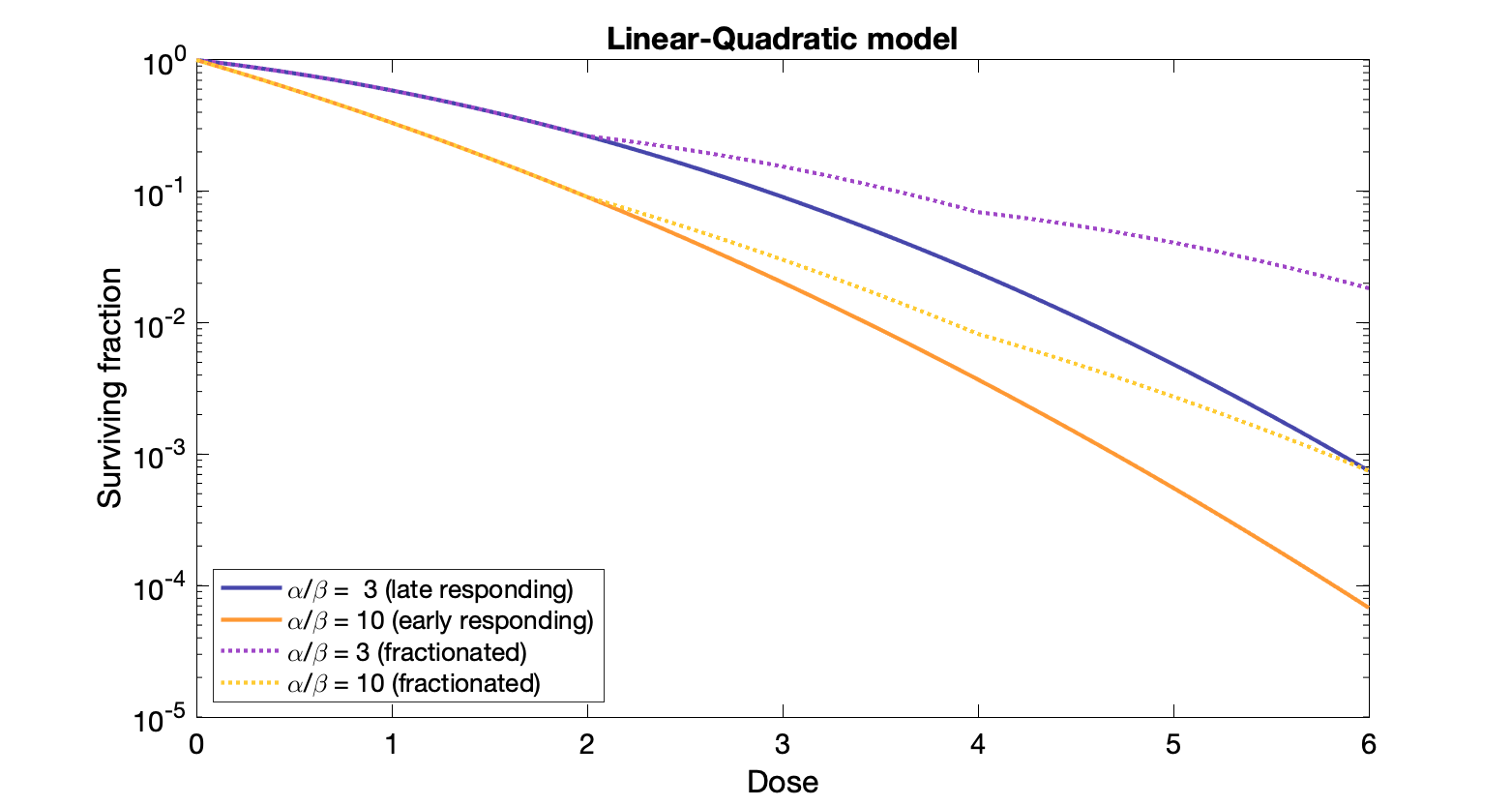
Linear-Quadratic Model (newer)
Fraction of cells $S$ surviving after a dose of D to tissue with constants α and β:
$S = e^{-\alpha D - \beta D^2}$
Dose at which linear and quadratic components are equal: $D = \alpha / \beta$
$y = \alpha D + \beta D^2$
Linear: single particle induces two adjacent breaks
Quadratic: two particles each create adjacent breaks.
Note: Only adequate for doses in typical radiation therapy. Higher doses the log survival becomes straight, but Linear-Quad model does not.
Ratio $\alpha / \beta$ is the dose at which linear and quadratic components of cell killing are equal.
Bystander effect
Biological effects in cells not directly traversed by ionizing radiation, but nearby. Small beam irradiations resulted in more aberrations that expected for beam and target.
Cytotoxic molecules presumably. Not just nucleus damage causing effects.
In vivo: "Abscopal" effects where cancer at distant site regresses after irradiation of a different site
Survival curve shape
Broad shoulder ⇒ radioresistant, low apoptosis (minimal laddering in gel electrophoresis)
Small shoulder ⇒ radiosensitive, high apoptosis (laddering)
Cancer stem cells more radioresistant than expected (usually resistance increases with differentiation).
Sensitivity can be increased by treatment with inhibitors of free radical scavengers.
Effective Survival Curve
For dose delivered in series of equal fractions with time between for repair of sublethal damage, the effective survival curve is an exponential function of dose (straight line on log-linear survival curve).
Many repeated shoulders add up.
$D_0$ is typically 3 Gy for normal human cells.
$D_{10} = 2.3 \times D_0$ which is the dose to kill 90% of the population (2.3 = ln(10)).
Calculations
$10^9$ cells dosed at 2 Gy and $D_0$ = 3.
90% chance of cure ⇒ depopulation of $10^{-10}$ (need less than 1 cell remaining?) ⇒ Need "10 decades of killing"
$10 \times D_{10} = 10 \times 2.3 \times D_0 = 10 \times 2.3 \times 3 = 10 \times 6.9 Gy = 69 Gy$
Rule of thumb: roughly $10^9$ cells in 1 cm3 volume.
Tumor control probability:
$\text{TCP} = e^{-(SF \times M)} = e^{-N} $, where SF is surviving fraction and M is number of clonogens and $N = SF \times M$ is the average number of surviving cells.
99% chance of cure → $N = SF \times M = 0.0101$
90% chance of cure → $N = SF \times M = 0.105$
50% chance of cure → $N = SF \times M = 0.693$
10% chance of cure → $N = SF \times M = 2.3$
Radiosensitivity and Cell Age
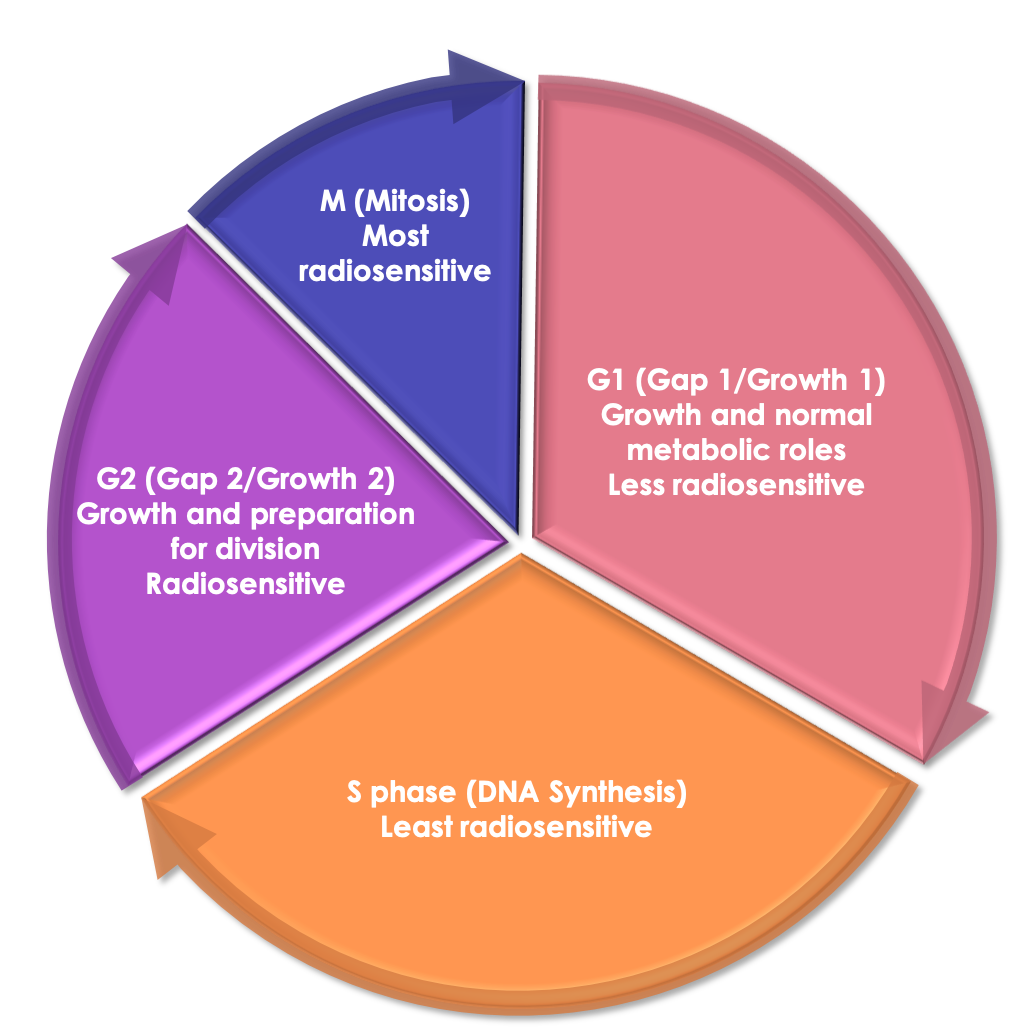
Cell cycle: mitosis (M), G1, DNA synthesis (S), G2, M, etc.
Cycle regulated by cyclin-dependent kinase (Cdk) family.
Cycle time from 9 hours (intestinal crypt cells) to 200 hours (mouse skin). G1 timing is most variable.
Radiosensitive: M and G2, NHEJ
Radioresistant: late S (two chromosome copies), and early G1 for long G1. HRR
OER slightly lower for cells in G1 than in S
Sensitive - M > G2 > G1 > early S > late S - Insensitive
Molecular checkpoints: stop cycle if DNA damage detected, (don't move to mitosis if chromosomes corrupted). Can be helpful to prevent mutations from being carried to daughter cells.
Fragmentation re-assorts sensitization
More rapidly dividing + lymphocytes (have high nuclear/cytoplasm ratio) → more radiosensitive
More | Lymphocytes → Spermatogonia → Myeloblasts → Crypt cells → Basal cells→ Endothelial → Gastric → Osteoblasts → Spermatocytes → Osteocytes → Erythrocytes → Fibrocytes → Chondrocytes→ Myocytes → Neurons | Less
Fractionation
Potentially lethal damage repair (PLD)
Post irradiation conditions can modify repair:
Preventing dividing (>= 6 hours), allows time for repair
PLD not significant for neutron irradiation
Sublethal damage repair (SLD)
Increase in survival if dose is fractionated
Half-time of SLD is 1 hour in mammals, longer in late-responding tissues
Repair of breaks before formation of chromosomal aberrations
SLD non-existent to neutrons
Dose-rate effect
Reduction in rate (e.g., 1 Gy/min to 0.3 Gy/hr) leads to reduction in cell killing because of SLD repair.
Lower dose rate, shallower survival curve ($D_0$ increases), less shoulder. (Opposite in some cell lines because lots are in G2)
Brachytherapy: Source dosage should reflect desired dose rate (e.g., 50-70 Gy in 5-9 days for an implant)
Oxygen Effects
Presence/absence of molecular oxygen affects the biological effect of x-rays ("fixates" damage)
Oxygen effect/enhancement ratio (OER): ratio of dose needed under hypoxic vs aerated conditions to produce same biologic effect.
Usually OER = 3 at high doses, and maybe around 2 at doses < 2 Gy.
Slightly lower in G2, slightly higher in S, intermediate in G1.
LET ↑ then OER ↓
Misonidazole - radiosensitizer that allows normally resistant hypoxic tumor cells to become sensitive (clinical trials show only slight benefit, except in specific cases). Limited dose due to toxicity.
Etanidazole and Nimorazole - less toxic versions, higher dose possible.
Biologically effective dose
$\text{BED} = nd(1+\frac{d}{\alpha/\beta})$, where $n$ is number of fractions and $d$ is dose per fraction.
Equivalent dose to 2 Gy/fraction
$\text{EQD2} = D\frac{d+\alpha/\beta}{2+\alpha/\beta}$ - new dose is total 2 Gy dose times ratio of (fraction dose + α/β)
Equivalent uniform dose
Two dose distributions are equivalent if the corresponding biological/clinical outcomes are equivalent.
$\text{EUD} = \left(\sum_i v_i D_i^a\right)^{1/a}$ where $v_i$ is the partial volume receiving dose $D_i$ and $a$ is tissue specific factor.
In normal tissue $a$ ranges from < 0.5 (parotids) to 20 (spinal cord) where higher $a$ means smaller volume effects (higher EUD with more weight given to areas of higher dose)
In tumors $a$ ranges from -13 (chordoma) to -7 (breast), thus much higher volume effects present.
$a$ is related to serial (high $a$) vs parallel (low $a$) organ function.
For tumors (negative $a$) the EUD will be between minimum dose and average dose. $a$→-∞, D → Dmin
For normal tissues (positive $a$) the EUD will be just below (a<1) or above average dose. $a$→+∞, D → Dmax
Fractionation: $\text{EUd} = \text{EUD}/n$ where $n$ is the number of fractions
Relevant for therapy techniques such as IMRT.
Late responding tissues: low α/β (equal killing, α=β, at lower dose), e.g., spinal cord.
Benefit from fractionation. Many cells in G0. Prolonging treatment times has small effect.
Larger shoulder on dose response plot, more curved
Early responding tissues: high α/β (similar to many tumors), e.g., skin, intestinal lining.
Prolonging treatment times is sparing (but also spare tumor, so would need to increase dose for tumor control)
Smaller shoulder dose response plot, less curved
Fraction size, total dose and overall treatment time determine acute effects/tumor control.
Accelerated fractionation to reduce repopulation, but increases acute effects. Little effect on late responding tissues.
Treatment time is not accounted for in BED (added proliferation effects).
Note that the difference between the surviving fraction for early and late responding tissues is greater in the fractionated case. Hence the sparing of normal tissue relative to the killing of tumor tissue, but also the need for more overall dose for the same amount of tumor control.
Cell culture calculations
Potential doubling time: $T_{pot} = \lambda T_s / LI$ where $\lambda$ is correction factor, $T_s$ is length of DNA synthetic period, $LI$ is labeling index.
$T_{pot} = \ln(2) / \ln(1 + GF) × T_c$ where $GF$ is growth factor, and $T_c$ is mean cell cycle time.
If cell loss, double time is : $T_D = T_{pot} / (1-\phi)$ where $\phi$ is cell loss factor
Law of Bergonie and Tribondeau
The radiosensitivity of tissue is increased the greater the number undifferentiated cells, the greater the mitotic activity, and the greater the length of time cells are actively proliferating.
4 R's of radiobiology
- Repair - time for normal cells to recover
- Repopulation - time for normal cells to divide
- Redistribution - cells in some phases (M, late G2) preferentially killed
- Reoxygenation - hypoxic regions of tumor become less hypoxic and more radiosensitive
Relative biological effectiveness (RBE): ratio of dose from 250 kV x-ray to dose from other radiation for the same effect.
E.g., protons have RBE of about 1.1 (maybe more at end of range). So 1.1 Gy of x-ray is needed for same biological effect as 1 Gy of proton. Related to LET, dose, # dose fractions, dose rate, biology.
Higher LET particles (α and neutrons), have denser columns of ionization.
LET ~ 2 keV/μm for 250-kV x-rays and ~166 keV/μm for 2.5-MeV α-particles
RBE starts to decrease at very high (>100 keV/m) LET. At 100 keV/m, separation of tracks is ~ 2nm = size of DNA double helix.
High LET, higher RBE for smaller dose/fraction.
RBE higher for cells with sublethal damage and repair (broad shoulder in survival curve).
Blood cells from more sensitive to least sensitive:
-Lymphocytes
-Red (erythrocytes)
-White (leukocytes)
-Platelets (thrombocytes)
Deterministic effects
Threshold dose for effects. Severity increases with dose. This is a concern for therapy applications and nuclear accidents.
Examples: radiation syndrome, cataracts, bone marrow ablation.
Timeline: prodromal radiation syndrome, latent period, manifest illness, recovery or death.
Severe prodromal response → poor prognosis (followed by acute hematologic aplasia, then anemia/hemorrhage)
Can use lymphocyte count to determine level of exposure.
LD50 ~ 5 Gy
High dose (~100 Gy), death in 24-48 hours, from cerebrovascular syndrome
Intermediate dose (5-12 Gy), death in 9-10 days, from gastrointestinal syndrome
Lowist dose (2-5 Gy), death in weeks to months, from hematopoietic syndrome
Timeline and causes depends on the population kinetics of cell renewal in the organ systems, and tolerance of damage.
- Prodromal radiation syndrome
- GI (anorexia, nausea, vomiting, diarrhea, intestinal cramps, salivation, fluid loss, dehydration, and weight loss)
- Neuromuscular (easy fatigability, apathy or listlessness, sweating, fever, headache, and hypotension)
- Latent stage
- No/lesser symptoms, length inversely proportional to dose
- Hematopoietic syndrome
- "bone marrow death", effects on the blood-forming organs
- delay in symptoms until circulating blood cells diminish and no new supply is provided
- Gastrointestinal syndrome
- bloody diarrhea and destruction of the gastrointestinal mucosa
- Cerebrovascular syndrome
- neurologic and cardiovascular breakdown
- nausea, vomiting, disorientation, loss of coordination, respiratory distress, seizures, coma, death
- causes not well understood
Treatment: antibiotics (low white cell counts, damaged intestines), not blood transfusions (delays regeneration of marrow),
Cataracts: opacification of eye lens. Dividing cells in pre-equatorial region of epithelium, differentiate in to lens fibers, and accrete at equator. Failure to differentiate leads to a cataract. No mechanism for removal of dead or damaged cells, so they accumulate. Latency ~ 8 years for dose 2.5-6 Gy. Threshold of 2 Gy single acute dose, 5-8 Gy if protracted exposure.
Stochastic effects
No threshold dose, probabilistic. Probability increases with dose, but not severity. This is the main concern for diagnostic applications, and also therapy (and nuclear accidents).
Example: hereditary damage, carcinogenesis, esp leukemia, thyroid cancer, lung cancer (uranium miners), bone cancer (radium dial painters), other solid tumors.
Radiation carcinogenesis
Damage to cell DNA may create mutations that lead to cancers.
Excess relative risk: rate of disease in an exposed population divided by the rate of disease in an unexposed population, minus 1.
Excess relative risk is estimated to be about 5.5 - 6% (entire population) and 4.1-4.8% (adults) per Sv. This percent is the risk of cancer above the natural risk (e.g. if natural risk is 40%, a Sv of radiation dose increases that risk to < 42.4%). Children are more susceptible to risks from radiation.
Heritable risk ~ 0.2%/Sv (ICRP)
Effects on the developing embryo
Effects depend on developmental stage (mostly studied from A-bomb survivors and from medical interventions):
- Pre-implantation: very sensitive (rapid proliferation). Malformations rare, spontaneous abortion more likely (all or nothing)
- Mice LD50 ~ 0.5 Gy
- Organogenesis: 10 - 41 days. Malformations, intrauterine growth retardation, decreased head diameter.
- Fetal period: 6+ weeks. Fewer abnormalities, microcephaly and mental retardation possible (esp. 8-15 weeks).]
- Severity of microcephaly increases with dose, and likelihood decreases after 16 weeks.
- Association between utero exposure and childhood cancer (causation uncertain).
- ~ 6%/Gy excess absolute risk (highly uncertain)
- if causative, increase cancer risk from year 10-15 by 50% (x-ray exam studies with confounding variables)
- typically leukemia
NCRP: maximum permissible dose (MPD) or 0.5 mSv/month
In USA, only regulated for radiation workers if pregnancy officially declared
< 10 cGy, no signification risks
Radiobiological basis of radiation protection
Want to avoid both deterministic and stochastic effects (see above).
Radiation increases risk of mutations but is a relative weak mutagen.
LD50 in humans: 4.5 Gy (total body)
Radiation worker limit: 50 mSv/yr (ALARA goal of 5 mSv/yr)
Public limit: 1-5 mSv/yr
Natural background: 3 mSv/yr
Average diagnostic exposure: ~ 3 mSv/yr
"Low dose" according to DOE is < 10 cGy/yr
Typically use a linear-no-threshold model, such that there is no "safe" dose level, and the chance of stochastic effects increases linearly with dose.
Models with threshold doses also proposed, but not generally adopted. Same for hormesis.
Excess risk depends on dose, age as time of exposure, time since exposure and gender.
Large uncertainties on risk for low doses
Radiation weighting factors WR: exposures to different types of radiation have different risks, based on biological effects.
WR: photons =1, electrons = 1, protons = 2, alphas = 20, neutrons = function of energy
Tissue weighting factors: accounts for susceptibility of tissue type to radiation. See table on radiation protection page